Abstract
Introduction Glycogen storage disease type Ib (GSDIb) and G6PC3-deficiency (G6PC3d) are two distinct genetic disorders associating neutropenia, bacterial infections susceptibility and inflamatory bowel disease (IBD), requiring GCSF therapy, and various immunotherapies. These neutropenias are caused either by a defect in the glucose-6-phosphate transporter of the ER or in the phosphatase G6PC3. These two defective proteins were recently shown to destroy a toxic metabolite, 1,5-anhydroglucitol-6-phosphate (1,5-AG6P), formed from the phosphorylation of a food derived polyol present in blood, 1,5-anhydroglucitol (1,5-AG), which otherwise accumulates in neutrophils, inhibits glycolysis and explains the neutropenia (Veiga-da-Cunha M, PMID: 30626647). As a direct result from this discovery, oral inhibitors of SGLT2 (iSGLT2s), the renal Na2+/glucose cotransporter 2 (e.g., empagliflozin or dapagliflozin) routinely used to treat type-2 diabetes were proposed to treat neutropenia in GSDIb and G6PC3d. Examples of such therapies have been successfully used in small cohorts of patients (Wortmann SB, PMID: 32294159; Boulanger, PMID: 35506446). We show preliminary results of the off-labelled study using iSGTL2s proposed to patients enrolled in the French severe chronic neutropenia registry (FSCNR).
Methods: Patients (or their parents) with a genetic diagnosis of GSDIb or of G6PC3d enrolled in the FSCNR, signed an informed consent to participate to this observational study. Empaglifozin or mostly dapaglifozin were started at a dose of 0.4 mg/kg/day or 0.2 mg/kg/day respectively. The study was divided in 3 phases. During the pre-therapeutic period, information was collected on the medical history and various biological parameters (complete blood counts, 1,5-AG, concentration, bone marrow smears). The initiation period aims to adapt the iSGLT2 dose while GCSF and immunotherapy were tapered down. The maintenance therapy was an observational therapy period, during which iSGLT2 dose remained largely unchanged.
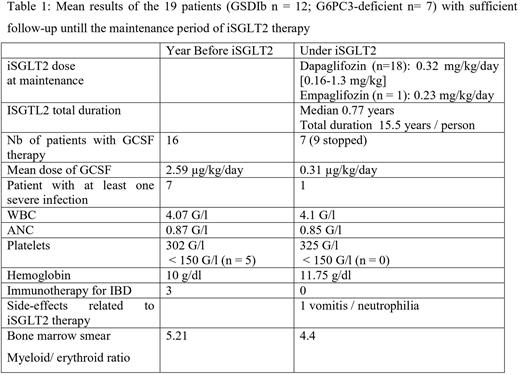
Results Twenty-one patients (GSDIb n = 14; G6PC3d n =7) were enrolled and the median follow-up was 0.8 years. The median age at the start of therapy was 18.8 years [range 0.4 - 37.7 years]. Two are still in the initiation period, while 19 are in the maintenance therapy and their results are summarized in the table 1. During the initiation period, 6 patients continued taking the initial iSGTL2 dose, while a dosage increased appeared necessary in 13 others, both to lower GCSF dose and to optimally reduce 1,5-AG in plasma which was particularly high at onset. The only severe side-effect was related to neutrophilia observed in a patient with G6PC3d with transient digestive manifestations (recurrent vomits and stomach pains). Patients with GSDIb required a longer initiation period to achieve a clinical benefit. Three patients were not taking GCSF during the pre-therapeutic period. For the 16 patients treated with GCSF prior to iSGLT2 therapy (iSGTL2t), the mean dose of GCSF was 2.5 µg/kg/day; 9 of them stopped GCSF, 6 decreased their daily dose and 1 was unchanged. Overall, the mean dose of GCSF under iSGTL2t was reduced by 8-fold to 0.31 µg/kg/day. During the pre-therapeutic period 3 patients were treated for severe IBD which required anti-TNF therapy (and surgery for 2). Most remarkably all 3 patients stop IBD related treatments, without recurrence of symptoms, while on iSGTL2t. Comparing periods, we observed limited and non-statistically significative change among hematological parameters (table 1). Of note, stable values for neutrophils were found while the GCSF was either tapered down or withdrawn. Bone marrow inspection indicated a qualitatively change related to the aspect of the endoplasmic reticulum in neutrophils which appeared less damaged both seen on their morphology and on the electronic microscopy images. Maturation arrest was never observed and myeloid compartiment appears less overstimulated under iSGTL2t.
Conclusion: iSGTL2s therapy in GSDIb and G6PC3d patients appeared to be a useful way to limit both GCSF therapy, infections and IBD manifestations. Initiation of iSGLT2s did not result in an instant effect. We often needed to adapt the dose of iSGTL2s both to spare GCSF therapy and to optimally lower 1,5-AG plasmatic level, indicating that its monitoring is useful to adapt the dosage. Further studies are required to define the best schedule of iSGTL2s in such subset of patients.
Disclosures
Marty:Incyte: Research Funding.
OffLabel Disclosure:
Empaglifozine and Dapaglifozine are Inhibitors of SGTL2 indicated for type 2 diabetes. In GSDib and G6PC3 neutropenia, they are proposed off label.
Author notes
Asterisk with author names denotes non-ASH members.